Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O -NaOH System)
-NaOH System)Yoshida, Eiichi; Aoto, Kazumi; Hirakawa, Yasushi;
JNC TN9400 2000-024, 42 Pages, 1999/10
For the purpose of improving the reliability of evaluation, the corrosion rate equation of the carbon steel SM400B (JIS G3106) in the high-temperature sodium compounds (NaOH-Na 0
0 system) was revised. ln this revision, the data acquired after 1997 was used. Based on the experimental results, the evaluation was made to be an approach to the following; (1)Metal loss of carbon steel in NaOH-Na
system) was revised. ln this revision, the data acquired after 1997 was used. Based on the experimental results, the evaluation was made to be an approach to the following; (1)Metal loss of carbon steel in NaOH-Na 0
0 system was evaluated as increases in exposure to the time, which is linear rate law. (2)There were no significant effects of the experiment atmosphere and mixing speed of the reagent on corrosion rate. (3)The concentration of Na
system was evaluated as increases in exposure to the time, which is linear rate law. (2)There were no significant effects of the experiment atmosphere and mixing speed of the reagent on corrosion rate. (3)The concentration of Na 0
0 in sodium compound is considered for the evaluation. The concentration under experiment is made to be the over concentration necessary for maintaining the dominant reaction between Fe and Na
in sodium compound is considered for the evaluation. The concentration under experiment is made to be the over concentration necessary for maintaining the dominant reaction between Fe and Na 20
20 . As a result of the evaluation, the additional data are 67 points. The data for the revision of the evaluation equation became the total of 105 points, when existing data of 38 points were added. The statistical evaluation of 105 points was carried out, and following recommended equation was obtained. C
. As a result of the evaluation, the additional data are 67 points. The data for the revision of the evaluation equation became the total of 105 points, when existing data of 38 points were added. The statistical evaluation of 105 points was carried out, and following recommended equation was obtained. C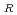 = C exp(-Q/RT) Where; C
= C exp(-Q/RT) Where; C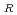 : Corrosion rate, mm/h C : Material constant Q : Apparent activation energy, cal/mol R : Gas constant, 1.986 cal/mol K T ; Absolute temperature, K Q = 9.61 kcal/mol C = 148.29 (average), 262.11 (99% UCL), 83.90 (99% LCL)
: Corrosion rate, mm/h C : Material constant Q : Apparent activation energy, cal/mol R : Gas constant, 1.986 cal/mol K T ; Absolute temperature, K Q = 9.61 kcal/mol C = 148.29 (average), 262.11 (99% UCL), 83.90 (99% LCL)